Inhibitors bind to BRAF(V600E)à block signalling
In BRAF(V600E) tumours, RAS is not activated, thus transactivation is minimal and ERK signalling is inhibited in cells exposed to RAF inhibitors.( BRAF inhibitor cannot induce the binding of BRAF to CRAF if BRAF is mutant)
Inhibitor binding activates WT RAF isoformà activate signalling
Drug mediated transactivation of RAF dimmers is responsible for paradoxical activation of the enzyme by inhibitors. Drug binding to one member of RAF homodimers or heterodimers inhibits one promoter, but results in transactivaiton of the drug-free protomer.
Induction of ERK signalling requires --- drug bind to ATP binding site of one kinase dimer
--- depend on RAS activity
-- Interaction with RAS-GTP àRas binding opens c-Raf to expose the docking site for mitogen-activated protein kinase kinase
c-Raf adopted two conformations: open active and closed inactive. Raf proteins adopt closed inactive conformation by means of a 14-3-3 dimer (fig 1A), which intramolecularly crosslinks the amino and carboxyl sides of the catalytic domains. Upon Ras activation, Raf proteins translocate to the plasma membrane, bind to Ras-GTP, and adopt an open conformation which enabled c-Raf to bind to MEK(fig 1B)
First, activation of c-Raf, Ras binding unfolds c-Raf to expose the docking site for MEK. Second, translocation of c-Raf to the plasma membrane was additionally required for the phosphorylation of MEK. (membrane localisation)
On open active sate, phosphorylation of several amino-acid residues including Ser 338, Tyr 341, Thr 491 and Ser 494 and/or lipid binding to c-Raf should occur at the plasma membrane to enable the phosphorylation of MEK, probably because these modifications are required for the activation of the catalytic domain.
Finally, induce dimerization of the cRAF.
--Induce dimerization
The RAF inhibitor GDC-0879 induced B-C and A-B heterodimers together with the induction of A-, B-and CRAF kinase activities.
RAF kinase inhibitors can induce ERK cascade signaling by promoting dimerization of RAF family members in the presence of oncogenic or normally activated RAS. This interaction is mediated by a dimer interface region in the RAF kinase domain called kinase suppressor of RAS (KSR).
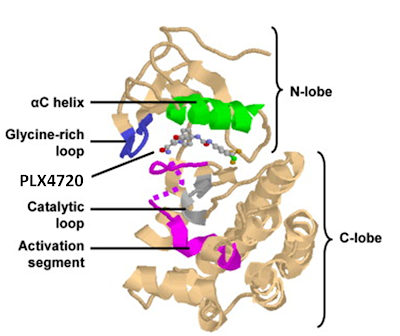
RAF catalytic function is regulated in response to a specific mode of dimerization of its kinase domain what is called the side-to-side dimer. (fig2) Moreover, KSR also participates in forming side-to-side heterodimers with RAF and can thereby trigger RAF activation. Intriguingly, kinase domain of BRAF was a monomer, in fact contains two RAF kinase domains that interact in a unique side-to-side fashion involving the N-lobe of their kinase domains. Fig a. Side-to-side dimerization of the RAF kinase domain provocatively engages helix αC. Helix αC of the RAF kinase domain adopts a productive conformation in the dimeric crystal configuration which is an active kinase conformation of RAF.
The catalytic activity of BRAF is triggered upon binding of two BRAF molecules to form a dimeric structure. Inhibitor bind to one BRAF molecule facilitate formation of the dimer, leading to transactivation of the second Raf molecule.(fig.3A) However high concentrations of inhibitor effectively block